Background: Emicizumab, a bispecific monoclonal antibody that bridges activated factor (F) IX and FX, substitutes for the function of deficient FVIII in people with hemophilia A (HA). Data from the Phase III HAVEN 6 trial (NCT04158648) show a favorable safety profile and efficacy of emicizumab in people with moderate or mild hemophilia A without FVIII inhibitors (Négrier et al. Lancet Haematol 2023). This analysis characterizes the dose and frequency of FVIII and tranexamic acid used for treatment of breakthrough bleeds in people with moderate or mild HA treated with emicizumab during HAVEN 6.
Methods: HAVEN 6 is a multicenter, open-label, single-arm, Phase III study designed to assess safety and efficacy of emicizumab in people with moderate or mild HA. The full study design has been published (Négrier et al.). Eligible participants included people of all ages with moderate or mild HA without inhibitors, who warranted prophylaxis as assessed by the investigator. Participants received a standard loading dose of emicizumab 3 mg/kg once weekly for 4 weeks, then a maintenance dose of 1.5 mg/kg once weekly, 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks. The lowest FVIII dose required to achieve hemostasis was recommended for breakthrough bleeds. Self-reported bleeds were defined as per the International Society on Thrombosis and Haemostasis (Blanchette et al. J Thromb Haemost 2014); treated bleeds excluded surgical bleeds. Tranexamic acid use in the 24 hours before a surgical procedure was excluded from this analysis. Inferential statistical analyses were not conducted.
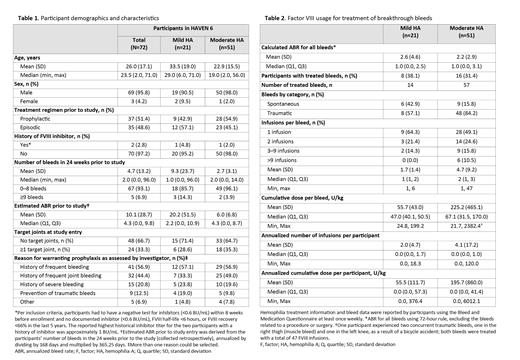
Results: At the data cut-off (October 30, 2021), 72 participants were enrolled; 51 (70.8%) had moderate HA and 21 (29.2%) had mild HA. Median (range) follow-up was 55.6 (8.7, 89.9) weeks.At baseline, 18 (35.2%) participants with moderate HA and 6 (28.5%) with mild HA had ≥1 target joint ( Table 1). The mean (SD) number of bleeds in the 24 weeks prior to the study was 2.7 (3.1) for those with moderate HA and 9.3 (23.7) for those with mild HA. During the HAVEN 6 study, 48 (66.7%) participants did not experience a treated bleed. Sixteen (31.4%) of the 51 participants with moderate HA and 8 (38.1%) of the 21 participants with mild HA received on-demand FVIII to treat a breakthrough bleed. All participants who experienced a bleed remained on emicizumab, regardless of additional FVIII usage. Of the 71 treated bleeds, 57 (80.3%) were in those with moderate HA and 14 (19.7%) were in those with mild HA ( Table 2). A total of 56 (78.9%) of the 71 treated bleeds were traumatic and 15 (21.1%) were spontaneous. Four participants experienced ≥6 treated bleeds during the study; 28 (90.3%) of the 31 bleeds experienced by these participants were traumatic. Overall, the median (range) number of FVIII infusions per treated bleed was 2 (1, 47) for participants with moderate HA and 1 (1, 6) for those with mild HA. Of the 71 treated bleeds, 54 (76.1%) required 1-2 FVIII infusions. Two participants, both aged 13 years and with moderate HA, had >9 infusions for a bleed. One participant had 19 infusions for a traumatic joint bleed on the finger/thumb. The other participant experienced two traumatic bleeds in the thigh/knee, treated with a total of 47 infusions. The same participant experienced a spontaneous thoracic bleed treated with 19 infusions, a traumatic joint bleed on the left ankle treated with 18 infusions, and a traumatic joint bleed in the finger/thumb treated with 13 infusions. The median (range) cumulative FVIII dose per bleed was 67.1 (31.5, 170.0) IU/kg for those with moderate HA and 47.0 (40.1, 50.5) IU/kg for those with mild HA. The mean (SD) annualized number of infusions per participant was 4.1 (17.2) for those with moderate HA and 2.0 (4.7) for those with mild HA. Seven (13.7%) people with moderate HA and 1 (4.8%) person with mild HA received tranexamic acid, ranging in duration from 1 to 7 days. Tranexamic acid was given for indications including fall with soft tissue bleed, nose bleeds, prophylaxis for dental procedure, and other. No adverse events, including thrombotic events, occurred with concomitant use of emicizumab and tranexamic acid. No desmopressin usage was recorded.
Conclusions: Reported bleeds were mainly traumatic and resolved after 1-2 doses of FVIII . No safety concerns were observed with concomitant use of emicizumab and FVIII and/or tranexamic acid. These data may inform breakthrough bleed treatment in people with moderate or mild HA receiving emicizumab.
Disclosures
Jiménez-Yuste:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Lim:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Mistry:Genentech, Inc.: Current Employment. Ventriglia:F. Hoffmann-La Roche Ltd.: Current Employment, Current holder of stock options in a privately-held company. Shapiro:Sanofi-Genzyme/Bioverativ: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Other: Clinical trial investigator ; Novo Nordisk Haemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; CSL-Behring: Membership on an entity's Board of Directors or advisory committees; Freeline: Other: Clinical trial investigator ; Indiana Hemophilia and Thrombosis Center: Current Employment; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial investigator .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal